Funded Programs
Synergy Oncology
Synergy Oncology
Synergy Spheres, a groundbreaking development in Selective Internal Radiation Therapy (SIRT), represent a significant leap forward in cancer treatment. These third- generation microspheres promise to redefine personalized cancer care, especially for extra-hepatic cancers. Featuring enhanced physical integrity and precise imaging capabilities, Synergy Spheres offer unparalleled delivery accuracy and real-time monitoring. Their adaptability to carry various therapeutic radionuclides allows for tailored treatment plans, optimizing outcomes across different cancer types.
Backed by CMIEDF funding, Synergy Oncology aims to refine and validate the product through rigorous laboratory testing, paving the way for clinical trials in 2025. This advancement holds the potential to revolutionize cancer treatment, offering hope to patients worldwide.

Yellowbird Diagnostics
Yellowbird Diagnostics creates new diagnostic imaging agents that address gaps in current clinical practices. Neuro- and cardio-inflammation are important indicators for a range of injuries and disease, however current radiotracers cannot adequately detect and map these inflammatory responses. Yellowbird’s first-to-clinic product is NeuCaVis, a unique radiotracer that, for the first time, can map fructolysis as an outcome of inflammatory disease.
Funding from CMIEDF will support the manufacturing and regulatory testing of NeuCaVis necessary to ensure a successful first-in-human trial. Ultimately, successful clinical translation will lead to earlier and more accurate diagnosis of neurological and cardiac diseases, enabling timely interventions, personalized treatment strategies, and development of new therapies.
Ac225 Bio Tx
Ac225 Bio Tx
With almost 2 million new diagnoses globally, and 850,000 deaths annually, and a 5- year survival of <10% (stage IV), colorectal cancer (CRC) treatment and management is a critically unmet clinical need. About 80% of CRC patients overexpress epidermal growth factor receptor (EGFR). The standard of care which includes the addition of an anti-EGFR antibody (e.g. cetuximab, panitumumab or nimotuzumab) to chemotherapy has a complete response rate of 0.5%. About 50% of patients do not qualify for treatment because they have mutations (KRAS and BRAF) in their CRC tumor cells.
ACT225 BioTherapeutics Corporation has preclinically validated an actinium-225 anti- EGFR antibody drug radioconjugate for targeted radiotherapy of EGFR-positive (wild- type and mutant) CRC. A zirconium-89 imaging pair has also been developed. The overall goal of the current funding is to develop the GMP biologic theranostic and complete clinical trial-enabling studies for potential phase 1 clinical trial.

ProteinQure
AI-driven optimization of a companion radiodiagnostic for a novel breast cancer therapeutic. Triple negative breast cancer (TNBC) presents significant treatment challenges, characterized by a 12% five-year survival rate, limited targeted therapy options, and invasive tests for treatment planning. Sortilin (SORT1), a cell surface and internalizing receptor overexpressed in various cancers, shows promise as a target for delivering high-potency, cytotoxic payloads or isotopes, aimed at maximizing toxicity towards cancer cells whilst minimizing it towards normal cells.
ProteinQure has a development candidate (PQ-203) for TNBC, currently in Investigational New Drug (IND) enabling studies. Our modular peptide-drug conjugate comprises a cytotoxic payload conjugated to a peptide targeting SORT1. Building on this, ProteinQure has initiated the development of a companion radiodiagnostic as part of the CMIEDF. Our project will optimize the peptide that could form the basis for a radiodiagnostic (and eventually a radiotherapeutic).

University of Ottawa Heart Institute (UOHI)
Detection of Apoptosis with 18F-Annexin Imaging in Carotid Atherosclerosis. We are developing a novel PET radiopharmaceutical for monitoring apoptosis in atherosclerosis. The basis of this technology is radiolabeling fluorine-18 to annexin V protein, which binds with high affinity to an extracellular membrane marker of apoptosis, phosphatidylserine. We have selected carotid atherosclerosis as a primary disease target due to the high prevalence of this condition and the availability of interventions for selection based on imaging-based risk stratification. The presence of significant apoptosis identified by 18F-annexin imaging in an atherosclerotic plaque may differentiate early and late atherosclerosis and guide clinical management.
Preclinical imaging is underway in mouse models of atherosclerosis. With CMIEDF funding, we will optimize radiopharmaceutical production for human imaging and complete Phase I and II clinical trials with PET imaging in healthy subjects and patients with advanced atherosclerosis.
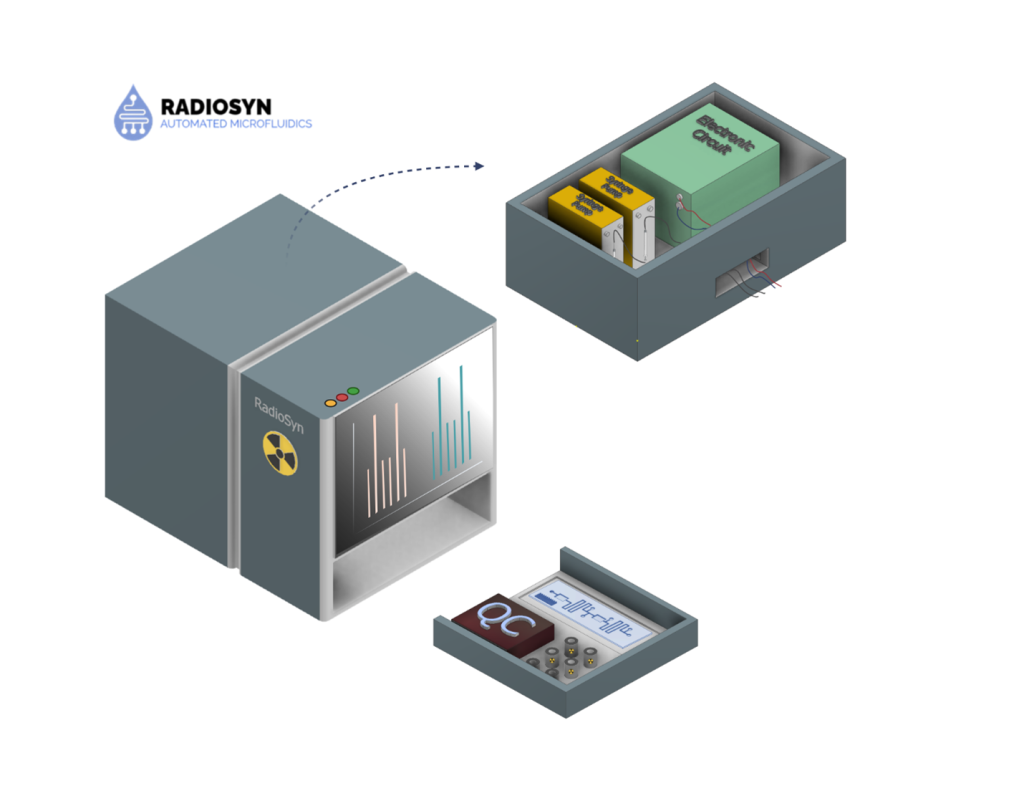
RadioSyn
RadioSyn is designing and manufacturing a miniaturized automated radiochemical synthesis platform. By integrating continuous flow microfluidic engineering with automated synthesis capabilities, RadioSyn delivers a shielded, state-of-the-art synthesizer that eliminates traditional production barriers and complexity. The platform uses disposable microfluidic chips that perform end-to-end chemical and radiochemical reactions. Our automated synthesis technology platform addresses critical limitations in conventional diagnostic and therapeutic workflows by standardizing and streamlining radioimmunoconjugate and radiopharmaceutical manufacturing. The Radiosyn platform offers reproducible, high-quality radiopharmaceutical production across research and clinical settings, requiring minimal specialized radiochemistry expertise. Our mission is to accelerate scientific discovery and clinical use of radiopharmaceuticals by making their production more accessible, standardized, and scalable.
RC14
RC14 is committed to supplying carbon-14, a vital material for drug development, environmental research, and the diagnosis of stomach ulcers. The ongoing geopolitical situation and sanctions have severely disrupted the carbon-14 supply, creating an urgent need for a reliable alternative.
To address this, RC14 is establishing a secure, Canadian-based supply of carbon-14. As an associate of CCNuclear, a respected provider of services to the Canadian nuclear industry, RC-14 will recycle carbon-14 from CANDU reactors waste stream. Through a three-step process, the isotope of interest will be recovered, refined, and repurposed, ensuring a steady supply for clients across North America, Europe, and Asia. The CMIE grant is designed to enable the manufacture of the commercial product, barium [14C]carbonate in Canada.
This Canadian initiative will support the advancement of new pharmaceuticals, improved agrochemicals, and continued medical diagnostics.
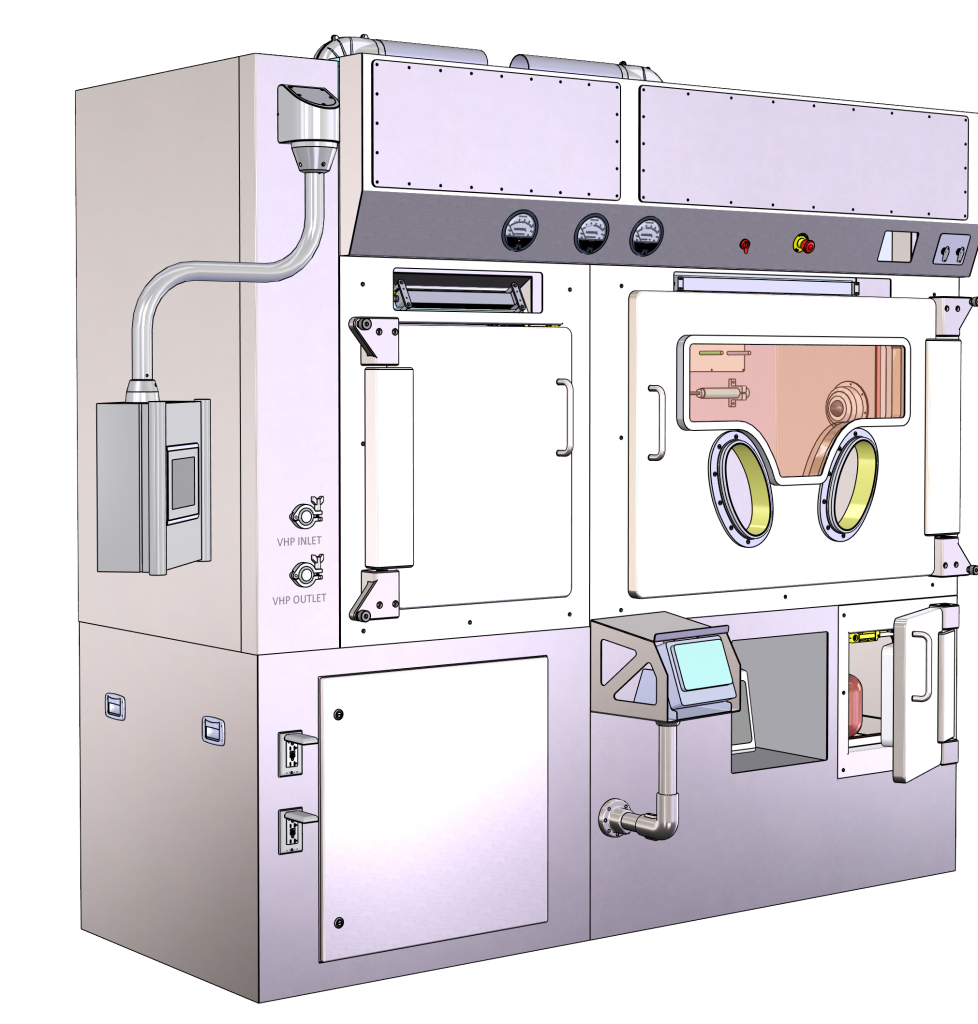
Promation
Promation is a Canadian company headquartered in Oakville, Ontario with a strong history of supporting the Canadian and global Automotive, Nuclear, and Radiopharmaceutical industries. Promation has proven experience in the Canadian
Medical Isotope Environment having provided custom hot cell automation solutions and secondary packaging lines for multiple installations including BWXT Medical’s Tc-99m line in Kanata, Ontario.
Canada’s radiopharmaceutical industry is experiencing rapid growth, but critical gaps in infrastructure limit its progress. Our project aims to address these limitations through the development of a Canadian-built, purpose-designed shielded isolator for GMP radiopharmaceutical applications. The support from the Canadian Medical Isotope Environment Development Fund (CMIEDF) will expedite this development, reducing dependence on the limited international suppliers and improving the quality and delivery timeline of such systems.
This shielded isolator will ensure timely and effective access to technical support, and address design challenges faced by Canadian producers. Moreover, it will serve as a catalyst for future projects supporting the Canadian Medical Isotope Environment including both Shielded Isolators and Hot Cells. The success of this project and subsequent projects will lead to the development of a more robust Canadian supply chain, a more competitive environment for isotope development and research and ultimately, better outcomes for Canadian patients who will have access to invaluable isotopes developed and manufactured here in Canada using Canadian equipment.
